
Even prior to that agreement, Bavarian Nordic and GRAM had begun working together on the technology transfer to enable the opening of a U.S.-based line.

On August 18, Bavarian Nordic, maker of JYNNEOS, and GRAM announced an agreement to establish this first U.S.-based fill and finish capability for the JYNNEOS vaccine, an agreement facilitated by BARDA. "This new agreement solidifies a domestic manufacturing capability that will bring us more vaccine sooner to end this outbreak." "We continue to build on our efforts to secure and make safe and effective vaccines readily available," said HHS Secretary Xavier Becerra. With BARDA's support, vaccine production at the facility is expected to be underway later this year, months ahead of the 9-month schedule typical for this type of work. The funding will allow GRAM to purchase additional equipment necessary for JYNNEOS production and recruit and train additional staff to operate the line. The agreement between the Biomedical Advanced Research and Development Authority ( BARDA), part of the HHS Administration for Strategic Preparedness and Response ( ASPR), and GRAM aids the company in accelerating the fill and finish manufacturing qualification and production in its recently expanded facility. Department of Health and Human Services (HHS) will provide approximately $11 million to support the first U.S.-based fill and finish manufacturing of JYNNEOS – a vaccine approved to prevent smallpox and monkeypox – at Grand River Aseptic Manufacturing (GRAM) in Grand Rapids, Michigan. We are actively seeking opportunities to further extend and capacitate COVID-19 vaccine manufacturing at this world class production facility.The U.S. “We are grateful for the opportunity to access this funding package… Aspen’s teams are working tirelessly to optimize production of the Johnson & Johnson COVID-19 vaccine for Africa at our manufacturing site in Gqeberha, South Africa. With this new facility, Aspen was able to offer Johnson & Johnson filling, finishing and packaging capacity for its COVID-19 vaccine, with the first batches having already been manufactured. Aspen recently built a fully certified sterile injectables facility at its existing site at Gqeberha, South Africa.
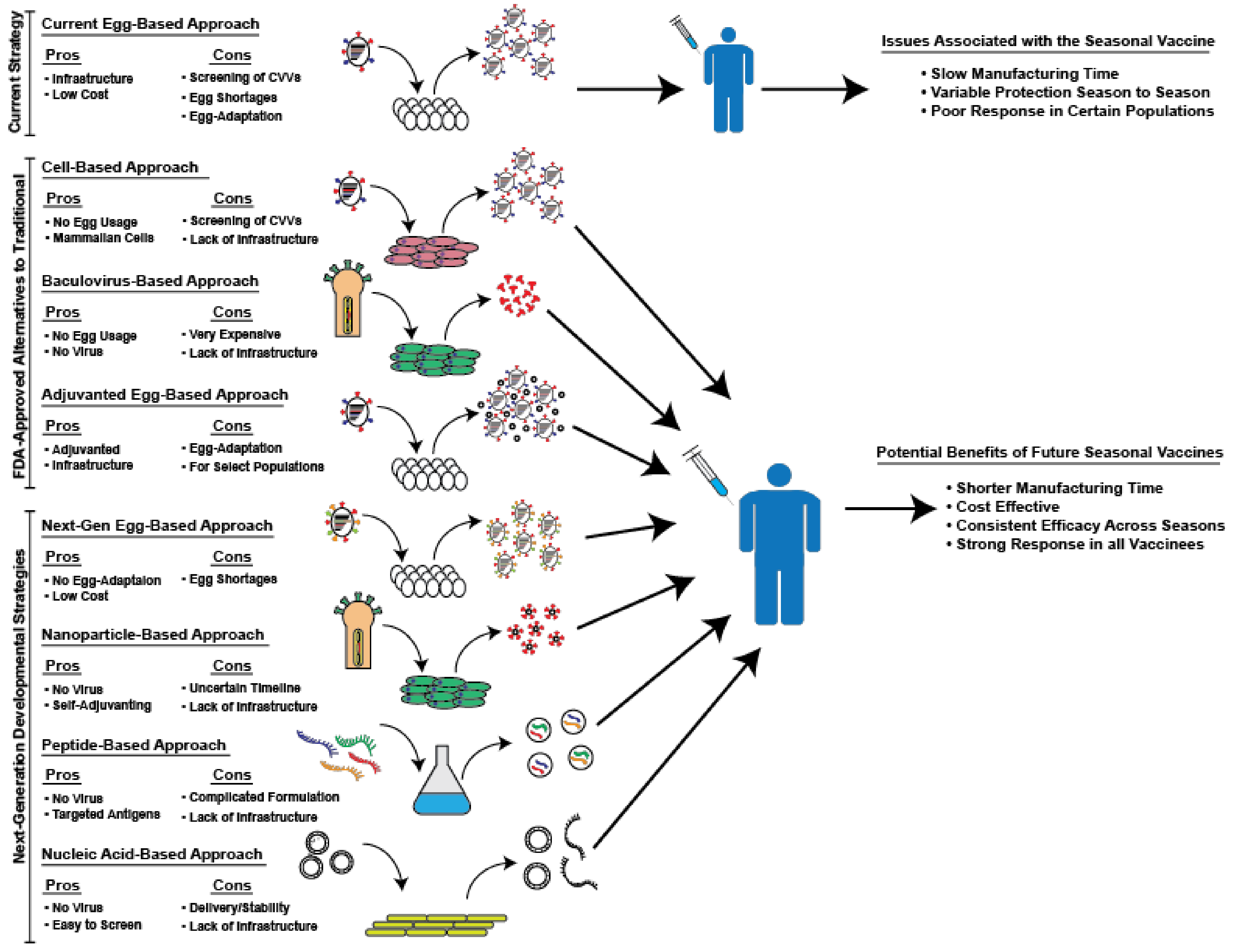
Aspen has partnered with Johnson & Johnson to compound, finish, fill and package the Janssen (a Johnson & Johnson company) COVID-19 vaccine at its sterile facility in South Africa. The €600 million long-term financing package will help Aspen, Africa’s largest pharmaceutical company, to refinance existing debt and strengthen the company’s balance sheet, supporting Aspen’s operations including production of vaccines, and other therapies in Africa and other emerging markets. Aspen, a leading pharmaceutical company in South Africa, is playing a major role producing COVID-19 treatment therapies and vaccines in Africa.

International Development Finance Corporation (DFC), IFC, the French Development institution Proparco, and DEG – the German development finance institution made a joint investment in Aspen Pharmacare Holdings Limited. Department of State Coordinator for Global COVID-19 Response and Health Security The Solution and Impact: It’s a short-term investment with a long-term vision.” But also, and importantly, as a long-term investment in the capacity of the continent to increase its own production of these vital goods so that there’s greater availability and resilience over time.
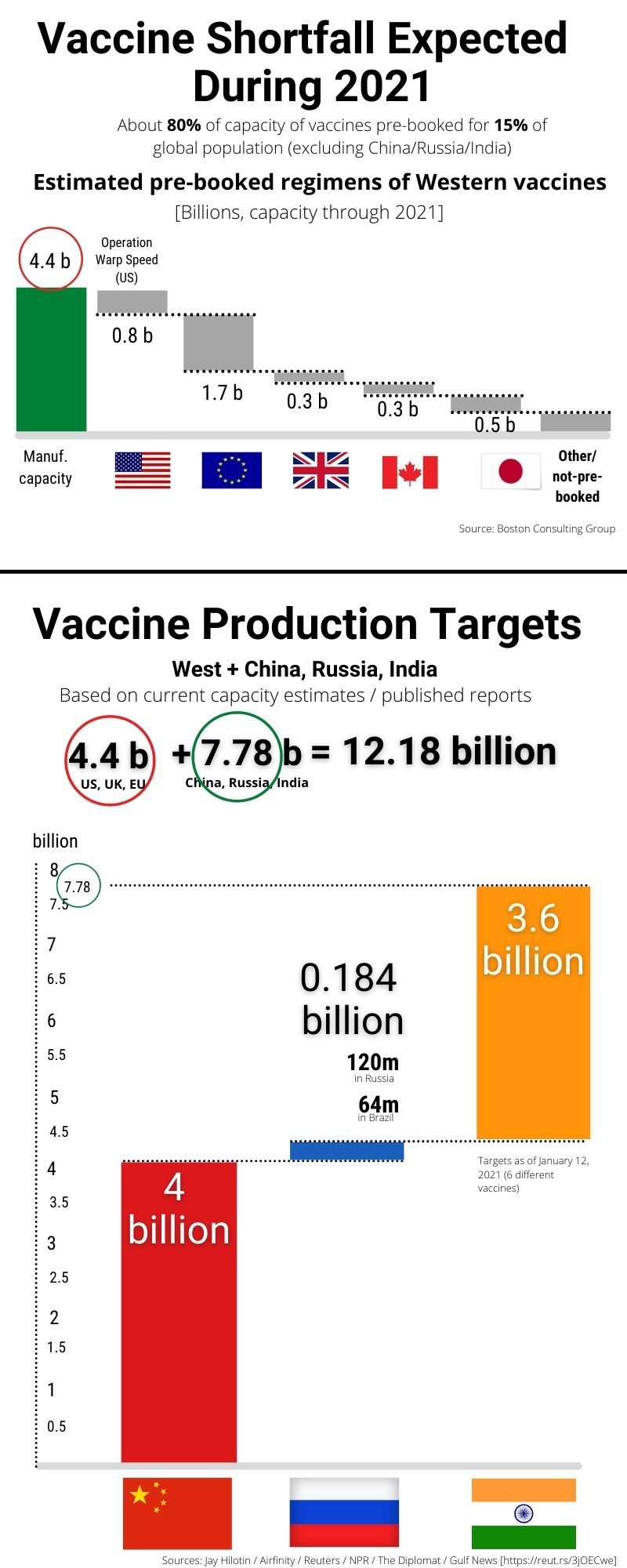
“We see this investment as, in the short term, a really valuable response to the urgent need on the continent for vaccines for COVID. This inequitable vaccine access not only leaves millions vulnerable to COVID-19 complications but also leaves the door open to variants that prolong the pandemic. In fact, as of August 2021, the Africa Centres for Disease Control and Prevention estimates that less than three percent of the continent’s population has completed their COVID-19 vaccination. While some countries have widescale vaccination efforts underway, the vast majority of people across the African continent have yet to receive a single shot. Vaccines help prevent serious illness, hospitalization, and death. Access to safe, effective vaccines is critical to ending the COVID-19 pandemic.


 0 kommentar(er)
0 kommentar(er)
